Abstract
BACKGROUND: Acute myeloid leukemia (AML) has a median age of diagnosis of 68 years; however, patients who are ineligible for intensive induction chemotherapy have limited therapeutic options. Venetoclax (Ven), an oral agent that targets the antiapoptotic protein, BCL-2, has shown synergistic antileukemic activity when combined with hypomethylating agents (HMA), decitabine (Dec) and azacitidine (Aza); this combination has resulted in high rates of durable remission, which are independent of cytogenetic and molecular characteristics.
METHODS: This is an open-label, phase 1b, dose escalation and expansion trial (NCT02203773) studying the safety and efficacy of venetoclax in combination with decitabine or azacitidine. Patients had previously untreated AML and were ineligible for intensive chemotherapy due to comorbidities and age. Here, we present the data from the expansion cohort, where patients were treated with 400 mg venetoclax in combination with either HMA. Venetoclax was administered daily in a three day ramp-up from 100 to 200 to 400 mg and coadministered with either 20 mg/m2 of intravenous (IV) decitabine on days 1-5 or 75 mg/m2 of IV or subcutaneous azacitidine on days 1-7 within each 28 day cycle. Dose adjustments for venetoclax were implemented for concomitant medications routinely used for prophylaxis with known drug-drug interactions. Safety and efficacy were evaluated. Time to first response, complete remission (CR), CR with incomplete blood count recovery (CRi), CR with partial hematologic recovery, duration of response, achievement of transfusion independence, overall survival (OS) and adverse events (AEs) were assessed. Minimal residual disease (MRD) was evaluated centrally by multicolor flow cytometry at a cutoff of 10-3 leukemic cells. Enrollment of patients treated with Ven + Aza began December 2014 and the majority was enrolled between January 2017 and June 2017; enrollment of patients treated with Ven + Dec occurred between November 2014 and June 2016, resulting in significantly different median follow-up time for the two treatment arms.
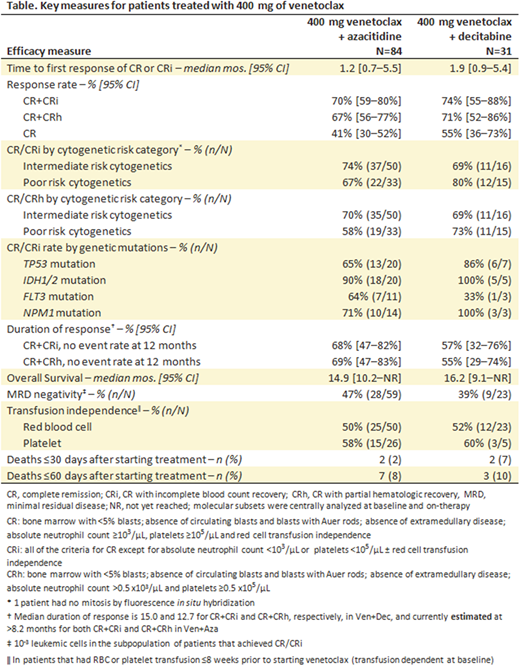
RESULTS: Data cutoff was December 22, 2017. Of 115 patients treated with the 400 mg dose of venetoclax, 84 were treated with Ven + Aza and 31 received Ven + Dec. The median ages for patients treated with Ven + Aza and Ven + Dec, respectively, were 75 (range: 61-90) and 72 (range: 65-86). Overall, 25% and 29% had secondary AML, and 39% and 48% had poor cytogenetic risk, in patients treated with Ven + Aza and Ven + Dec, respectively. Transfusion dependence for red blood cells (RBC) or platelets within 8 weeks prior to venetoclax treatment was 64% (54/81) and 74% (23/31) in patients treated with Ven + Aza and Ven + Dec, respectively. Key grade ≥3 AEs across all patients were febrile neutropenia (44%), anemia (28%), pneumonia (25%), thrombocytopenia (22%) and neutropenia (18%). Median time on study treatment was 6.4 and 5.7 months, and median follow up was 8.2 (range: 0.4-35.5) and 16.2 (range: 0.7-36.7) months for patients treated with Ven + Aza and Ven + Dec, respectively. Key efficacy results are shown in the Table. Seventy percent and 74% of patients achieved CR/CRi, and the median time to first response was 1.2 and 1.9 months for patients treated with Ven + Aza and Ven + Dec, respectively. The median overall survival was 14.9 months for patients treated with Ven + Aza and 16.2 months for those treated with Ven + Dec. Among patients transfusion dependent at baseline, 52% (40/77) achieved transfusion independence from both RBC and platelets, defined as not receiving RBC or platelet transfusion for ≥56 days. Across both treatment groups, among patients with CR/CRi, 45% achieved MRD response less than 10-3 leukemic cells. Patients who received venetoclax dose reduction for CYP3A inhibitors had similar responses compared to those without dose reduction.
CONCLUSIONS: Venetoclax in combination with either azacitidine or decitabine led to high rates of rapid and deep responses that were durable in patients with AML ineligible for standard induction chemotherapy. A majority of patients who were transfusion dependent at baseline achieved transfusion independence after initiating venetoclax therapy. These results suggest that venetoclax, in combination with hypomethylating agents, may provide a potent therapeutic option for patients with AML who are not eligible for intensive chemotherapy.
Pollyea:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy; Curis: Membership on an entity's Board of Directors or advisory committees. Pratz:AbbVie: Consultancy, Research Funding; Agios: Research Funding; Astellas: Consultancy, Research Funding; Boston Scientific: Consultancy; Millenium/Takeda: Research Funding. Letai:AbbVie: Consultancy, Other: Lab research report; AstraZeneca: Consultancy, Other: Lab research report; Novartis: Consultancy, Other: Lab research report; Vivid Biosciences: Equity Ownership; Flash Therapeutics: Equity Ownership. Wei:Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Konopleva:Stemline Therapeutics: Research Funding. Frankfurt:AbbVie: Membership on an entity's Board of Directors or advisory committees; Celgene, Jazz, Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rizzieri:GlaxoSmithKline: Research Funding. Xu:AbbVie, Inc: Employment, Equity Ownership. Dail:Genentech: Employment, Equity Ownership. Chyla:AbbVie, Inc: Employment, Equity Ownership. Potluri:AbbVie: Employment, Equity Ownership. DiNardo:Celgene: Honoraria; Agios: Consultancy; Karyopharm: Honoraria; Abbvie: Honoraria; Medimmune: Honoraria; Bayer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal